Search Case Studies
End-Use Case Study
- RTP Company’s Expertise and Tech Support Help Produce Radiopaque Catheter
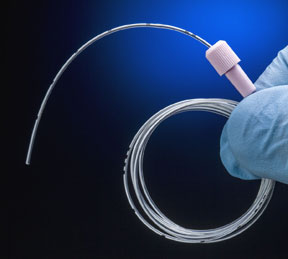
Recently, PyMPSA’s design & development department assigned engineer Claudia Mancilla Ramirez to work with PiSA on developing a new catheter for epidural anesthesia. The specs called for a biocompatible nylon material that was radiopaque so it could be readily observed during x-ray imaging to ensure proper placement.
Their first efforts, explained engineer Mancilla, were disappointing for the quality-conscious company.
“With our original supplier, the extruded nylon ended up with a lot of air bubbles, so it was far from meeting the quality criteria we and our customers demand,” she said.
In search of a new material supplier, the PyMPSA team met representatives from RTP Company at a medical device trade show. The difference, they say, was significant.
“RTP Company listened, and not only developed a custom material for our application, they backed it up with technical support. They sent an extrusion technician here that worked side-by-side with our extrusion engineer Lily Díaz to ensure production of a high quality product,” said engineer Mancilla.
With RTP Company’s assistance, PyMPSA solved the air bubble problem, leading to the successful development of a new epidural catheter that is marketed by PiSA under its SET ESPICAT brand. The catheter is manufactured using a custom RTP 2900 Series polyether-block-amide thermoplastic elastomer (PEBA) compound that includes radio pacifiers and passed ISO 10993 biocompatibility testing conducted by PyMPSA.
“In our experience, this level of service is uncommon for suppliers,” noted engineer Mancilla. “RTP Company’s attitude was ‘we can help.’ When we needed information and assistance they responded quite well and quite fast. Combine that with an excellent product, and it led to a very successful first project together.”
Indeed, engineers Mancilla and Díaz noted the success of the catheter project has already led PyMPSA to reach out to RTP Company on several additional new products. They are also working together to produce the catheter in several different colors to help medical and manufacturing personnel quickly identify different diameter versions.
“Now that we know of RTP Company’s capabilities, we look forward to many more successful projects with them in the future,” said engineer Mancilla.
Plásticos y Materias Primas is part of the PiSA Farmacéutica Mexicana group of companies who are Mexico’s leaders in healthcare. For more information visit the website for either firm at www.pympsa.com.mx or www.pisa.com.mx.




